L-Monoamine oxidases (MAO) are a family of enzymes that catalyze the oxidation of monoamines. They are found bound to the outer membrane of mitochondria in most cell types in the body. The enzyme was originally discovered by Mary Bernheim (maiden name: Hare) in the liver and was named tyramine oxidase. They belong to the protein family of flavin-containing amine oxidoreductases.
Subtypes and tissue distribution
Both are found in neurons and astroglia.
Outside the central nervous system:
MAO-A is also found in the liver, gastrointestinal tract, and placenta.
MAO-B is mostly found in blood platelets.
Function
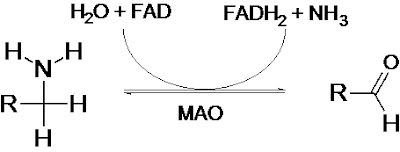
Monoamine oxidases catalyze the oxidative deamination of monoamines. Oxygen is used to remove an amine group from a molecule, resulting in the corresponding aldehyde and ammonia. The general form of the catalyzed reaction (with R denoting an arbitrary group) is:
Monoamine oxidases contain the covalently-bound cofactor FAD and are, thus, classified as flavoproteins.
Substrate specificities
Serotonin, melatonin, norepinephrine, and epinephrine are mainly broken down by MAO-A.
Phenethylamine is mainly broken down by MAO-B.
Both forms break down dopamine equally.
Specific reactions catalyzed by MAO include:
Epinephrine or norepinephrine to 3,4-Dihydroxymandelic acid
Metanephrine or normetanephrine to vanillylmandelic acid (VMA)
Dopamine to dihydroxyphenylacetic acid
3-Methoxytyramine to homovanillic acid
Genetics
A study reported in Science in August 2002 based on the Dunedin cohort concluded that maltreated children with a low-activity polymorphism in the promoter region of the MAO-A gene were more likely to develop antisocial conduct disorders than maltreated children with the high-activity variant. Out of the 442 total males in the study (maltreated or not), 37% had the low activity variant. Of the 13 maltreated males with low MAO-A activity, 11 had been assessed as exhibiting adolescent conduct disorder and 4 were convicted for violent offenses. The suggested mechanism for this effect is the decreased ability of those with low MAO-A activity to quickly degrade norepinephrine, the synaptic neurotransmitter involved in sympathetic arousal and rage. This is alleged to provide direct support for the idea that genetic susceptibility to disease is not determined at birth, but varies with exposure to environmental influences. Note however that most of those with conduct disorder or convictions did not have low activity of MAO-A; maltreatment was found to have caused stronger predisposition for antisocial behavior than differences in MAO-A activity.
Research also uncovered a possible link between predisposition to novelty seeking and a genotype of the MAO-A gene.
In 2006, a New Zealand researcher, Dr Rod Lea said that a particular variant (or genotype) was over-represented in Māori, a Warrior gene. This supported earlier studies finding different proportions of variants in different ethnic groups. This is the case for many genetic variants, with 33% White/Non-Hispanic, 61% Asian/Pacific Islanders having the low-activity MAO-A promoter variant.
Clinical signifance
Because of the vital role that MAOs play in the inactivation of neurotransmitters, MAO dysfunction (too much or too little MAO activity) is thought to be responsible for a number of neurological disorders. For example, unusually high or low levels of MAOs in the body have been associated with depression,schizophrenia, substance abuse, attention deficit disorder, migraines, and irregular sexual maturation. Monoamine oxidase inhibitors are one of the major classes of drug prescribed for the treatment of depression, although they are last-line treatment due to risk of the drug's interaction with diet or other drugs. Excessive levels of catecholamines (epinephrine, norepinephrine, and dopamine) may lead to a hypertensive crisis, and excessive levels of serotonin may lead to serotonin syndrome.
PET research has shown that MAO is also heavily depleted by use of tobacco cigarettes.