Products of the feeding, drink and medicine, which follows to avoid when receiving inhibitor MAO
The Following products of the feeding, drink and medicine follows to avoid when receiving inhibitor MAO and during fortnight after their cancelling:
Meat and fish
Meat prepared with preservative
Meat extracts
Smoked or pickled fish
Govyazhiya or chicken liver
Dry sausages, salami
Vegetables and fruits
Canned dates
Pod broad bob
Bananas and avocado (particularly overripe)
Milk products
Cheese and cheese containing products such as cheese cookie and pizza (are permitted pressed pot cheese and cream cheeses)
Yogurt and sour cream
Drink
Beer, red wine and other alcoholic drink
Miscellaneous
Soya sauce
Yeast extract (including beer yeast in greater amount)
Overweening amount of chocolate and caffeine
Not fresh, bad or long kept, a second time processed protein products such as meat, fish and milk products
Medicinal facilities
Tablets or mixtures from cold, hay fever or sinusitis
Remedies for head cold (tablets, dripped or aerosols)
Ingalyacionnye remedy for asthmas
Facilities for reduction of the appetite
Facilitators
Drugs, including cocaine
Coaxil, description of the preparation
The Trade name:
Coaxil
The International name:
Tianeptine
The Group attribute:
Antidepressant
Description acting material:
Tianeptine
The Medicinal form:
pills covered by shell
Uses of Coaxil:
Warnings and precautions for Coaxil
This medicine should not be used during pregnancy or breast-feeding.
This medicine should not be used: With MAO inhibitors (as a rule, there should be an interval of 15 days between a MAOI and Tianeptine treatments) By expectant or nursing mothers By children of less than 15 years of age Should you have any doubt, consult your doctor.
Side Effects of Coaxil:
Gastralgia,
abdominal pain,
dryness of the mouth,
anorexia,
nausea,
vomiting,
flatulence Insomnia,
drowsiness,
nightmares,
asthenia Tachycardia,
extrasystole,
precordialgia Dizziness,
headaches,
faintness,
trembling,
upsets Respiratory discomfort,
tightness of the throat Myalgia,
lumbago
Any of the above mentioned should be reported to your doctor or chemist in addition to any adverse effects not mentioned in this leaflet.
How to Take Coaxil
Take this medicine as directed. It can be taken with or without food. Do not stop taking this medicine abruptly without consulting with your doctor.What to do if you take Overdose of Coaxil?
Seek medical attention immediately. For non emergencies, contact your local or regional poison control center.What to do if you take Missed Dose of Coaxil?
Take your next dose as soon as you remember. If it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take a double dose to make up for a missed one.How to Store Coaxil:
Keep this medication in the container it came in, tightly closed, and out of the reach of children. Store it at room temperature and away from excess heat and moisture (not in the bathroom). Throw away any medication that is outdated or no longer needed. Talk to your pharmacist about the proper disposal of your medication.The Description of the preparation coaxil is not intended for purpose of the treatment without participation of the physician.
Monoamine oxidases (MAO)
L-Monoamine oxidases (MAO) are a family of enzymes that catalyze the oxidation of monoamines. They are found bound to the outer membrane of mitochondria in most cell types in the body. The enzyme was originally discovered by Mary Bernheim (maiden name: Hare) in the liver and was named tyramine oxidase. They belong to the protein family of flavin-containing amine oxidoreductases.
Subtypes and tissue distribution
Both are found in neurons and astroglia.
Outside the central nervous system:
MAO-A is also found in the liver, gastrointestinal tract, and placenta.
MAO-B is mostly found in blood platelets.
Function
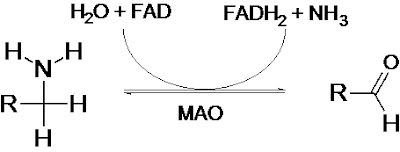
Monoamine oxidases catalyze the oxidative deamination of monoamines. Oxygen is used to remove an amine group from a molecule, resulting in the corresponding aldehyde and ammonia. The general form of the catalyzed reaction (with R denoting an arbitrary group) is:
Monoamine oxidases contain the covalently-bound cofactor FAD and are, thus, classified as flavoproteins.
Substrate specificities
Serotonin, melatonin, norepinephrine, and epinephrine are mainly broken down by MAO-A.
Phenethylamine is mainly broken down by MAO-B.
Both forms break down dopamine equally.
Specific reactions catalyzed by MAO include:
Epinephrine or norepinephrine to 3,4-Dihydroxymandelic acid
Metanephrine or normetanephrine to vanillylmandelic acid (VMA)
Dopamine to dihydroxyphenylacetic acid
3-Methoxytyramine to homovanillic acid
Genetics
A study reported in Science in August 2002 based on the Dunedin cohort concluded that maltreated children with a low-activity polymorphism in the promoter region of the MAO-A gene were more likely to develop antisocial conduct disorders than maltreated children with the high-activity variant. Out of the 442 total males in the study (maltreated or not), 37% had the low activity variant. Of the 13 maltreated males with low MAO-A activity, 11 had been assessed as exhibiting adolescent conduct disorder and 4 were convicted for violent offenses. The suggested mechanism for this effect is the decreased ability of those with low MAO-A activity to quickly degrade norepinephrine, the synaptic neurotransmitter involved in sympathetic arousal and rage. This is alleged to provide direct support for the idea that genetic susceptibility to disease is not determined at birth, but varies with exposure to environmental influences. Note however that most of those with conduct disorder or convictions did not have low activity of MAO-A; maltreatment was found to have caused stronger predisposition for antisocial behavior than differences in MAO-A activity.
Research also uncovered a possible link between predisposition to novelty seeking and a genotype of the MAO-A gene.
In 2006, a New Zealand researcher, Dr Rod Lea said that a particular variant (or genotype) was over-represented in Māori, a Warrior gene. This supported earlier studies finding different proportions of variants in different ethnic groups. This is the case for many genetic variants, with 33% White/Non-Hispanic, 61% Asian/Pacific Islanders having the low-activity MAO-A promoter variant.
Clinical signifance
Because of the vital role that MAOs play in the inactivation of neurotransmitters, MAO dysfunction (too much or too little MAO activity) is thought to be responsible for a number of neurological disorders. For example, unusually high or low levels of MAOs in the body have been associated with depression,schizophrenia, substance abuse, attention deficit disorder, migraines, and irregular sexual maturation. Monoamine oxidase inhibitors are one of the major classes of drug prescribed for the treatment of depression, although they are last-line treatment due to risk of the drug's interaction with diet or other drugs. Excessive levels of catecholamines (epinephrine, norepinephrine, and dopamine) may lead to a hypertensive crisis, and excessive levels of serotonin may lead to serotonin syndrome.
PET research has shown that MAO is also heavily depleted by use of tobacco cigarettes.
Tianeptine
Tianeptine (INN) (Stablon, Coaxil, Tatinol) is a drug used for treating major depressive episodes (mild, moderate, or severe). It has structural similarities to the tricyclic antidepressants, but it has different pharmacological properties. Until recently, it has been assumed that tianeptine is a selective serotonin reuptake enhancer (SSRE), opposite to the action of SSRIs. However, newer studies question this hypothesis; one review suggests that long-term administration of tianeptine has no effect on serotonin pathways, while another still points to the cancellative effects of tianeptine and fluoxetine coadministration on serotonin reuptake. Tianeptine enhances the extracellular concentration of dopamine in the nucleus accumbens and modulates the D2 and D3 dopamine receptors, but this effect is modest and almost certainly indirect. There is also action on the NMDA and AMPA receptors. Recent reviews point to this pathway as a hypothesized mechanism of action, based on tianeptine's effect of promoting stress-associated impaired neuroplasticity.
Tianeptine reduces the effects of serotonin in the limbic system and the pre-frontal cortex, giving rise to a mood elevation, unlike the mood blunting associated with SSRIs.[citation needed] Like SSRIs, however, tianeptine's onset-of-action delay is approximately 2–6 weeks with improvements sometimes noticeable in as soon as one week. Its short-lived, but pleasant, stimulant effect experienced by some patients is shared with its predecessor, amineptine, whose side effects related to dopamine uptake inhibitor activity resulted in Servier's research into tianeptine.[citation needed] Suggested dosage is three times daily, due to its short duration of action.
Tianeptine has strong antidepressant and anxiolytic properties with a relative lack of sedative, anticholinergic and cardiovascular adverse effects, thus suggesting it is particularly suitable for use in elderly patients and in those following alcohol withdrawal; such patients can be more sensitive to the adverse effects of psychotropic drugs. Recent interesting results indicate anticonvulsant and analgesic activity of tianeptine and its possible interaction with adenosine A1 receptors.
Mechanism of action
In contrast to SSRIs and tricyclic antidepressants, tianeptine modestly enhances the mesolimbic release of dopamine, but it is also unclear how this occurs because tiapentine itself has no effect on dopamine transporters, nor does it affect D1, D2, D3, D4 and D5 receptors.
Perhaps the most studied hypothesis is that Tianeptine has a protective effect against stress induced neuronal remodeling. This is all based largely on preclinical studies.
Abuse and addiction potential
One patient reportedly consumed a total of 240 12.5 mg tablets (3000 mg) per day for several months and was later successfully detoxified in an inpatient setting. The report indicated that a tolerance was developed and there were physical withdrawal symptoms.
In 2007, according to French Health Products Safety Agency, tianeptine's manufacturer Servier agreed to modify the drug's label, following problems with dependency.
Singapore's Ministry of Health has restricted the use of tianeptine to psychiatrists due to its abuse potential, while Bahrain has classified it a controlled substance due to increasing reports of misuse and abuse by patients.
Tianeptine (under "Coaxil" brand name) has been intravenously injected by drug users in Armenia and Russia. This method of administration reportedly causes an opioid-like effect and is sometimes used in an attempt to lessen opioid withdrawal symptoms. As tianeptine tablets do not fully dissolve and often the solution is not filtered well, particles in the injected fluid can block small blood vessels, leading to thrombosis and then severe necrosis.
Approved
Investigational and ongoing research
It is currently being researched for its effectiveness in irritable bowel syndrome.
Tinaneptine has been found to be effective in depression in Parkinson's disease and in post-traumatic stress disorder of which it was as safe and effective as fluoxetine (Prozac, an SSRI) and moclobemide (Aurorix, an MAO-A inhibitor).
Tablets coaxil
Coaxil
See also Tianeptine
Roman name
Coaxil
Of drugs
Antidepressive, anxiolytic, reducing the pool, improved mood, mild propulsion delayed, the overall tone, good conduct (including alcoholic abstinence).
Indications
Тreating depression weak moderately to seriously.
Patient interaction
Koaksil not apply in combination with MAO inhibitors, which is in danger of collapse or sudden arterial hypertension, gipertermii, convulsing; It is also possible fatal outcome.
Overdosing
In all cases overdose should suspend treatment Koaksilom and closely monitor the patient (with the work of the heart and lungs, kidney function, metabolism). If necessary, rinse the stomach and the symptomatic treatment (particularly IVL, and the correction of the kidney and violations of metabolism).
Storage conditions
List B. The temperature is not above 30 ° C.
Shelf life
3 years
Subscribe to:
Posts (Atom)